Results/summary will be near the bottom of this post!
I've tried API, Sera, and Salifert test kits for a while now, and I found that I "liked" using the Salifert kits the most. The test kits usually aligned with my observations of plant health and algae growth. Remember, healthy plants don't grow algae, and plants can't be healthy if they don't have every single element available to them in some form. If I have a decrease or issue with CO2 injection, I get staghorn/hair algae on my plants. If I bottom out on NO3, I get hair algae or cyanobacteria on the plants. If I bottom out on PO4, I get GSA (especially on the glass and large-leaved plants). If I bottom out on K, well, plants stop growing altogether, and I often get GDA or other algae on my plants.

You can see on the right door my little selection of different test kits that I use. Journal for this tank here.
Testing gives me an idea as to whether I'm in the "zone" for growing plants (which is basically anything greater than 0ppm). It's not foolproof, but it's one of the best tools and I've found it to be SERIOUSLY enlightening to help me understand what I'm observing. I'm now to the point that I can often guess correctly if a certain nutrient has bottomed out simply based on what changes my plants are going through. I usually test once every week or two, but these are high energy systems. If something is bottomed out/out of balance, I'll pay the price very quickly compared to low-tech, which I can imagine requires far less frequent testing to gain an understanding of the system.
Dry salt fertilizers aren't always the most pure. and even a little dissolved in some RO water massively increases the ppm beyond what these test kits use. For example, to increase 1 gallon of RO water to 50ppm NO3, you need to add 0.309g KNO3. That's WAY too small of an amount to be accurately measured (even by my precision 0.0001g lab scale), especially because there could happen to be some impurities in my powder sample that would have a massive effect at only 0.309g.

You can see random dark spots, flecks of grey/black, and other potential impurities in my KNO3 from GLA ferts. This isn't a problem for the amounts we actually use in DIY fertilizer solutions, or dry fert dosing, but it is a problem for testing the accuracy of test kits.
So, I need to make a large enough "stock" concentrate solution that I'm using a "sizeable" amount of the dry salts to avoid impurity issues and measurement issues. Then , I'll mathematically dilute the concentrated stock to a known usable/testable solution.
This only works with pure RO water. My RO water is about 0-2TDS and should work fine. I'll be testing not just one test kit each, but two per nutrient! I bought 9 kits total, 3 of each lol
The math:
Formula is C1*V1=C2*V2, where:C1= concentration of stock,
V1= volume of stock to be diluted in RO water later,
C2= desired concentration of diluted solution,
V2= desired volume of diluted solution.
Solve for V1:
V1=(C2*V2)/C1
Then: V2-V1= volume of pure RO water to add V1 to to make your diluted solution.
Example: Dilute some volume (V1) of a 300ppm unusable concentrate into 100mL of a usable 10ppm solution:
Dissolve 1.85g KNO3 in 3,785.4g (1gal) RO water to make a 300ppm concentrate stock.
V1 = (10ppm*100mL)/300ppm = 3.333mL concentrate
RO water needed for diluted solution: (100mL desired volume V2 ) - (3.333mL) = 96.667mL RO water needed
Answer: Dilute 3.333mL of the 300ppm NO3 concentrate solution in 96.667mL RO water to create 100mL of a 10ppm NO3 solution.
The work:

Start with 1 gallon of RO water (3,785.4g).

Into the 1 gallon RO water, dissolve 1.85g KNO3 and stir without splashing/removing water. You now have a 300ppm NO3 concentrate.

Measure 96.667mL pure RO water.
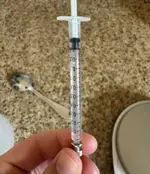
Using a precise syringe (like this 1.0mL syringe) add 3.33mL of the concentrate to the pure RO water to make 100mL total at 10ppm concentration.
Now, follow the instructions for the Salifert Freshwater NO3 test kit: 2mL water sample in vial, 4 drops of NO3-1 (make sure you hold vertically when dropping, always!), swirl 10s+, then 1 level scoop of NO3-2. I use the soft sides of the container to compress the scoop into a very level scoop:

Swirl for 30 seconds, then start the 3-minute timer:

After 3 minutes, read top-down, ideally with natural light:

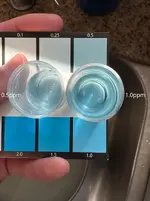
That's pretty bang-on for 10ppm NO3!
I repeated the process a second time and got 10ppm. I had my partner come take a look, and she said 10ppm for both results.
I then tried it with a second kit as well:

Also got 10ppm (hard to see in this photo).
However, it's important to note with this salifert test kit (and many No3 test kits out there):
The results change if you let them sit for too long. I found that 3.5 minutes post-swirl had the most spot on results for 10ppm, but if I let the vial sit for even 6 or 10 minutes, the results changed quickly:

After 6 minutes it looked like 20ppm.

After 15 minutes, it looked like 40ppm.
The instructions clearly state to take a reading after 3 minutes, but they don't emphasize this enough. You have approx 30-60 seconds after the 3-minute post-swirl timer to get a correct reading. Not a problem or even an issue at all, just something important to know. API nitrate also does this, btw.
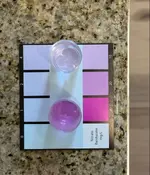
Here are two vials, both at 10ppm NO3. The top is fresh off the 3-minute timer, while the bottom sat for over 20 minutes. Again, super accurate @ 10ppm as long as you take the reading between 3-4 minutes post-swirl.
Then, I tried a 40ppm solution to test the high range of the solution. After 3 minutes with test kit 1, here's the results:

Test kit 1 results @ 40ppm

test kit 2 results @ 40ppm
Findings for Salifert Freshwater NO3:
With known 10ppm solution:Kit 1, #1: 10ppm
Kit 1, #2: 10ppm
Kit 2, #1: 10ppm
Kit 2, #2: 10ppm
With known 40ppm solution:
Kit 1, #1: 40ppm
Kit 1, #2: 40ppm (almost looked closer to 30ppm, but not by much)
Kit 2, #1: 40ppm
Kit 2, #2: 40ppm
I'd say that between these two different kits (all reagents within expiration date) this gets my seal of approval. That's very accurate for a liquid test kit.
Results:
After testing two different NO3 test kits at two different NO3 concentrations (med-low and high) it appears to be very accurate (at least between 10-40ppm).Make sure you are packing the NO3-2 mini spoon flat/level with powder. Swirl hard for 30 seconds and set a strict 3 minute timer.
Readings may be most accurate at 3.5 minutes. Read top down. Very accurate, 9/10 would recommend.
Last edited: